Introduction
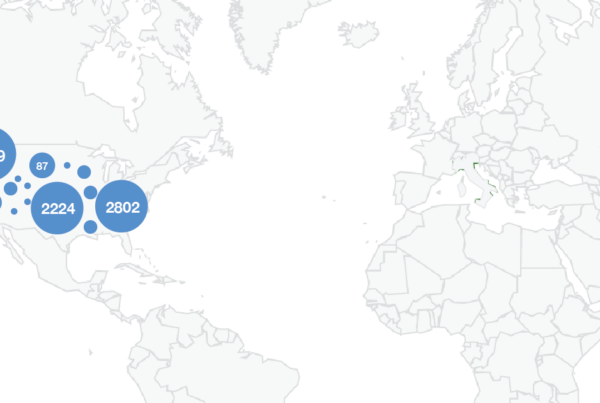
The FDA recently published a set of 5.6 million medical device reports previously hidden from the public. These “Alternative Summary Reports” (ASR) constitute over 40% of the total device reports now available.
The FDA characterized data from these hidden reports as containing “well-known and well-established risks” with the implication that the information within was of limited value. Our analysis suggests the opposite
One of the Food and Drug Administration’s core responsibilities is to collect and publish reports describing medical device problems and adverse events. This real-world data can help inform patients, doctors, and researchers as to potential risks to patient health, and weigh the comparative cost-benefit tradeoffs of similar devices.