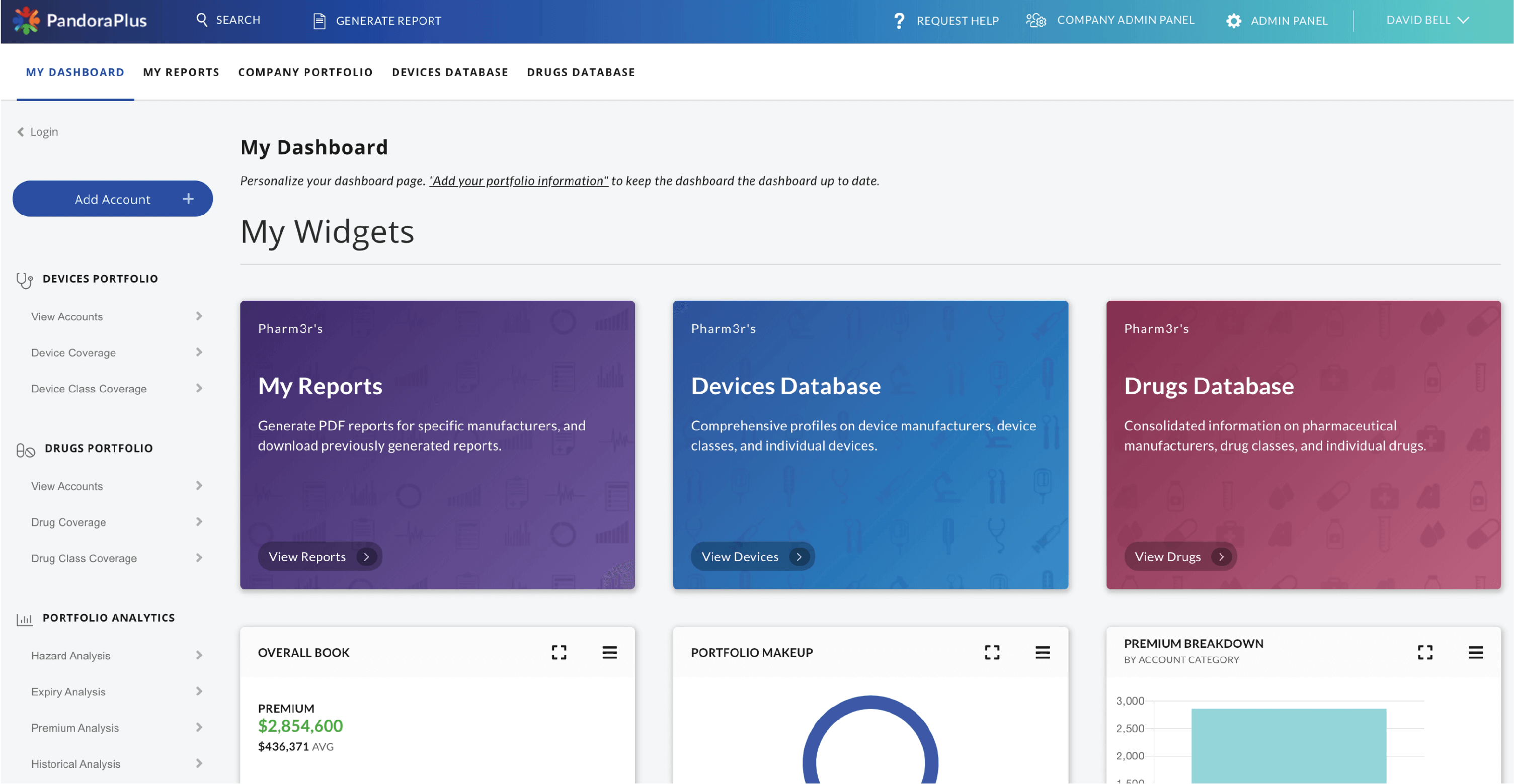
Risk Assessment, Comparative Analytics, and Trend Awareness
PandoraPlus offers far more than just data aggregation.
It provides in-depth insight into medical product risk and quality. At-a-glance risk assessment, historical trend analysis, prospective safety measures, and best-in-class comparisons make PandoraPlus the most powerful post-market surveillance tool available.
Drugs and devices are browseable by product class, and PDF report generation and portfolio management are also available.
- Our data universe encompasses over 50,000 medical device and drug companies, and millions of medical products.
- Rapid intuitive views by product class, generic type, brand name, and manufacturer.
- Know the exact content of MAUDE narratives
- Export reports to PDF to get a quick view of the most pressing issues
- Analyze Drug Accountability by NDA Ownership
- Know the warnings and adverse events related with each label change and event history
Before PandoraPlus
Teams would spend weeks and months researching to find medical product risks and quality information. The data collected was disparate, often incomplete, and missing pertinent relationships in order to make valuable insights trusted by the organization.
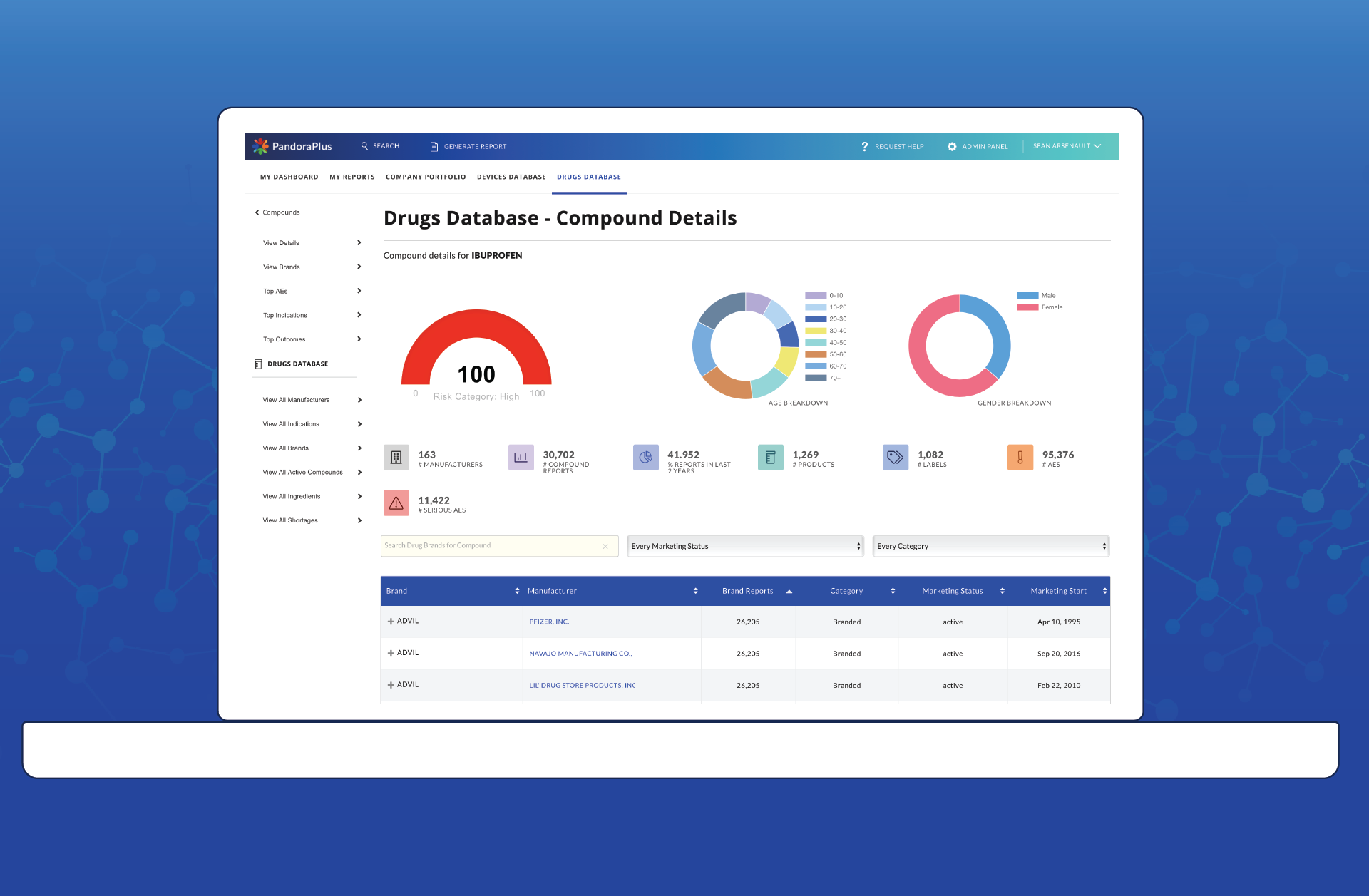
With PandoraPlus
At-a-glance risk assessment, historical trend analysis, prospective safety measures, and best-in-class comparisons make PandoraPlus the most powerful post market surveillance tool available. Within minutes customers are able to turn data assets into data insights, allowing them to make rapid informed decisions that are reliable.
